Multiple sclerosis (MS) is a disease that affects millions worldwide. It happens when the immune system mistakenly attacks the protective covering of nerves in the brain and spinal cord, leading to symptoms such as fatigue, muscle weakness, difficulty walking, numbness, and even problems with memory and thinking.
Over the years, treatments have helped many patients manage relapses and slow disease activity. But for people with progressive MS, options remain very limited, and a cure is still out of reach.
Now though, several new therapies are entering clinical trials in the United States, bringing fresh hope to the MS community.
What’s New in MS Treatment Research?
Traditionally, MS drugs have worked by calming down the immune system to reduce inflammation. The therapies now being tested go further, aiming not only to control the immune system but also to repair damage and prevent long-term disability. Here are three of the most exciting approaches:
1. CAR-T Cell Therapy – Reprogramming the Immune System
In August 2025, a patient in Nebraska became the first person worldwide to receive a CAR-T cell therapy designed for MS.
This treatment uses special T cells (a type of immune cell) that are genetically engineered to “reset” the faulty immune response driving MS.
Early trials are small and focused mainly on safety, but if successful, this approach could offer longer-lasting benefits than current therapies.
2. BTK Inhibitors – Slowing Disability in Progressive MS
A new class of oral drugs called BTK inhibitors is showing promise, especially for progressive MS.
Tolebrutinib, one of the most advanced in this group, was found to reduce disability progression by 31% in a major Phase III trial.
This is significant news because progressive MS often continues to worsen despite existing treatments.
3. Remyelination Therapies – Repairing Nerve Damage
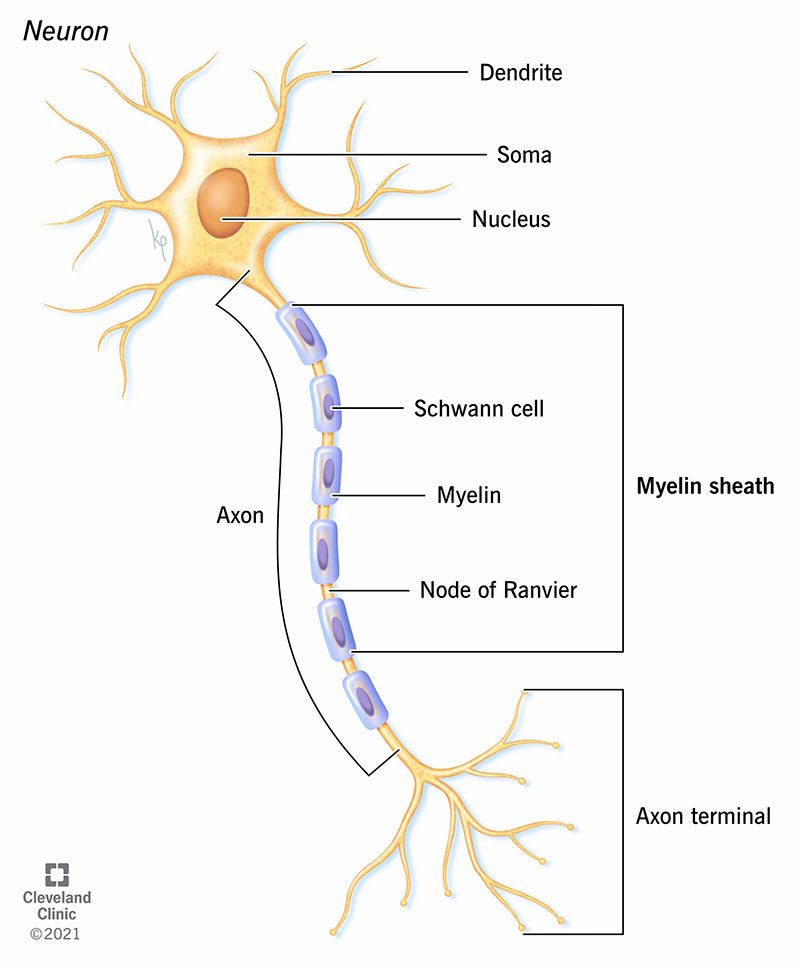
Researchers are also testing drugs that may help the body repair damaged myelin (the protective nerve covering destroyed in MS).
A trial combining two common medicines — metformin (a diabetes drug) and clemastine (an allergy medicine) — showed small signs of myelin repair.
While results are still early, this represents a completely new way of thinking about MS treatment: not just stopping attacks, but fixing damage.
Why This Matters
For patients and families, these developments mean:
New hope for progressive MS, where options have been limited.
The possibility of longer-term control of the disease, beyond relapse prevention.
Early evidence that repairing nerve damage may one day be possible.
But it’s also important to remember:
These treatments are still in early trials, and safety is the top priority.
It may take several years before we know whether they are safe, effective, and widely available.
Not all patients may qualify for every new therapy.
A Step Toward the Future
The launch of U.S. clinical trials marks a critical step forward. While we must stay realistic — clinical research is slow and full of challenges — the MS community can be encouraged that the scientific spotlight is firmly focused on finding better answers.
Every trial brings us closer to the ultimate goal: a world where people with MS can live their lives free from disability and fear of progression.
Takeaway: MS research is entering a bold new phase. With immune-resetting cell therapies, powerful new drugs, and even attempts to repair myelin, the landscape is changing. Patients, caregivers, and advocates should watch these trials closely — the next few years could reshape how we think about MS treatment.
References
- https://www.nejm.org/doi/full/10.1056/NEJMoa2415988?utm_
- https://www.theguardian.com/society/2025/sep/26/exciting-clinical-results-offer-hope-for-new-class-of-ms-therapies-multiple-sclerosis?utm_
- https://www.unmc.edu/newsroom/2025/08/06/nebraska-medicine-patient-is-first-to-receive-new-ms-therapy/?utm_

